Overview of TTC-352 Attributes

Novel mechanism of action for hormonal therapy of breast cancer

Effective in heavily-pretreated ER+ breast cancer after failure of hormonal therapy and CDK4/6 inhibitors

Biomarker predicting activity in development (PKCa overexpression)

Oral capsule delivery

Human safety established

Significant key opinion leaders support for novel, non-toxic breast cancer therapy
Our Solution: TTC-352

TTC Oncology introduces TTC-352, a selective human estrogen receptor partial agonist (ShERPA). TTC-352 is designed to treat metastatic ER+ breast cancers that have failed on first-line endocrine therapy and a CDK4/6 inhibitor. TTC-352 is expected to be effective in cases where breast cancer patients develop resistance to endocrine therapy (about 625,000 patients).
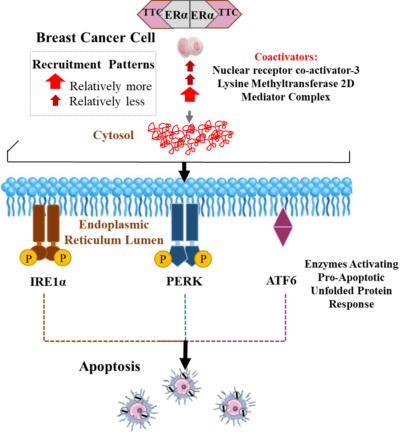
TTC-352 builds on the demonstrated clinical efficacy of estradiol in breast cancer, with a mechanism of action that is similar to estradiol in causing tumor regression in resistant cancers, but is predicted to abrogate the side effects associated with estradiol. These side effects in gynecologic tissues have severely limited the use of a therapeutic approach known by many key opinion leaders (KOLs) to be effective in the clinic and to serve an unmet need in metastatic ER+ breast cancer.
Clinical Trial Results

Completed phase I clinical trial confirmed that the drug is safe and demonstrated encouraging efficacy in patients with refractory ER+ breast cancer that is heavily pretreated with hormonal and chemotherapy refractory breast cancer.

In addition, a biomarker of benefit has been identified (PKCa overexpression).
