Overview of TTC-352 Attributes

Novel mechanism of action for hormonal therapy of breast cancer

Effective in heavily-pretreated ER+ breast cancer after failure of hormonal therapy and CDK4/6 inhibitors

Biomarker predicting activity in development (PKCa overexpression)

Oral capsule delivery

Human safety established

Significant key opinion leaders support for novel, non-toxic breast cancer therapy
Our Solution: TTC-352

TTC-352 is an orally-administered selective human estrogen receptor (ER) partial agonist (ShERPA) designed to treat metastatic ER+ breast cancers that have become resistant to endocrine therapy and CDK4/6 inhibitor therapy.
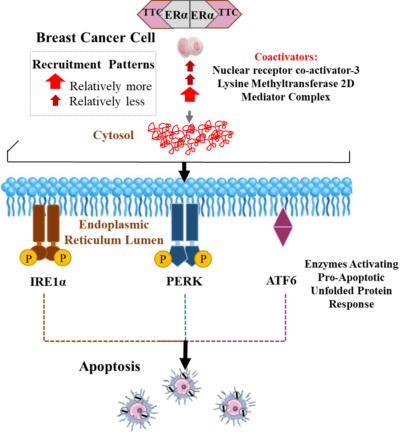
TTC-352 builds on the mechanism of action of estradiol, interacting with estrogen receptor on tumor cells leading to tumor regression, but without the magnitude of side effects.
Clinical Trial Results

Completed phase I clinical trial confirmed that the drug is safe and demonstrated encouraging efficacy in patients with refractory ER+ breast cancer that is heavily pretreated with hormonal and chemotherapy refractory breast cancer.

In addition, a biomarker of benefit has been identified (PKCa overexpression).